Page 39 - ESHRE2019
P. 39
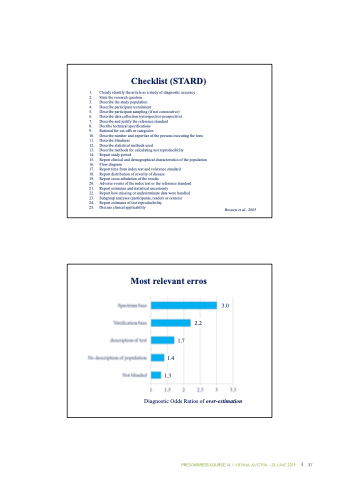
Checklist (STARD)
1. Clearly identify the article as a study of diagnostic accuracy
2. State the research question
3. Describe the study population
4. Describe participant recruitment
5. Describe participant sampling (if not consecutive)
6. Describe data collection (retrospective-prospective)
7. Describe and justify the reference standard
8. Decribe technical specifications
9. Rational for cut-offs or categories
10. Describe number and expertise of the persons executing the tests
11. Describe blindness
12. Describe statistical methods used
13. Describe methods for calculating test reproducibility
14. Report study period
15. Report clinical and demographical characteristics of the population
16. Flow diagram
17. Report time from index test and reference standard
18. Report distribution of severity of disease
19. Report cross-tabulation of the results
20. Adverse events of the index test or the reference standard
21. Report estimates and statistical uncertainty
22. Report how missing or undeterminate data were handled
23. Subgroup analyses (participants, readers or centers)
24. Report estimates of test reproducibility
25. Discuss clinical applicability
Bossuyt et al., 2003
Most relevant erros
3.0 2.2
1.7 1.4
1.3
Diagnostic Odds Ratios of over-estimation
34
PRECONGRESS COURSE 14 I VIENNA, AUSTRIA – 23 JUNE 2019 37