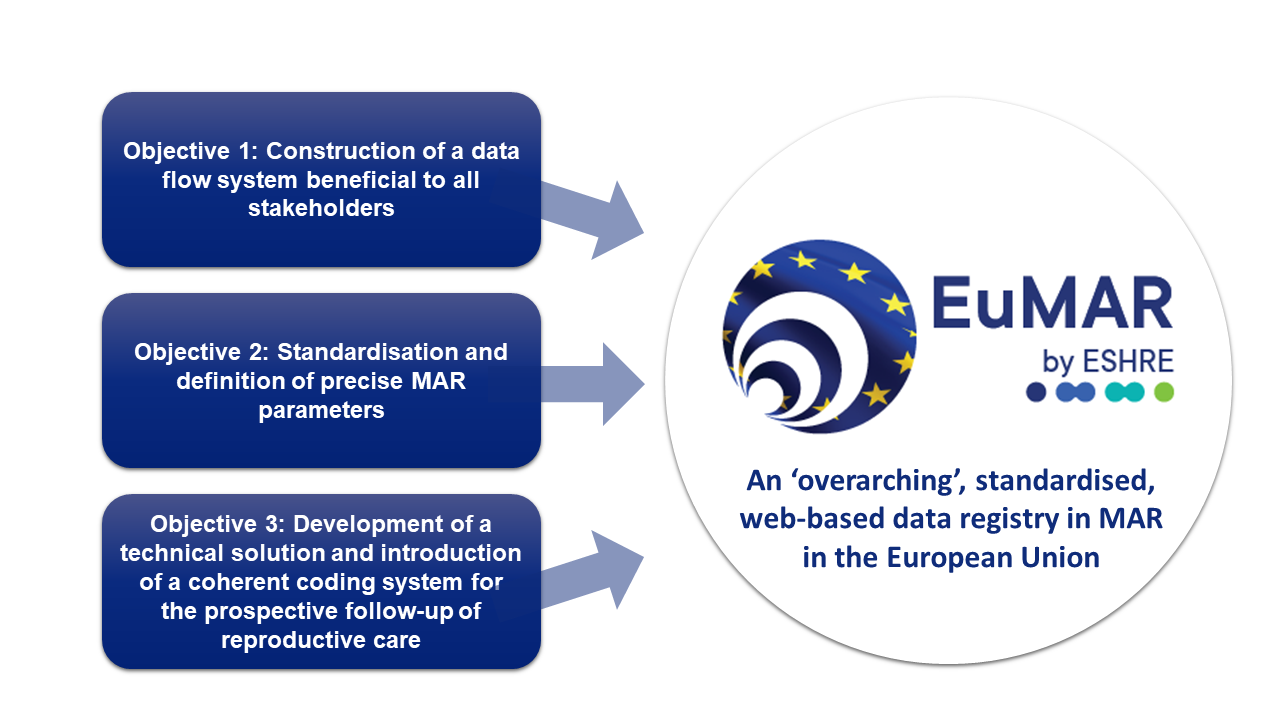
European monitoring of Medically Assisted Reproduction
EuMAR is a three-year project (2023-2025), co-funded by the EU4Health program at the European Commission (DG SANTE) and run by ESHRE. Its aim is to develop a pan-European registry of prospective cycle-by-cycle data on the use and outcomes of medically assisted reproduction (MAR) treatments. EuMAR addresses the need for:
• Transparency and accessibility of data
• Quality assurance and surveillance
• Standardisation of parameters and definitions for comparability of data
• Flexibility to connect to other registries in the future
• The possibility to calculate cumulative outcomes and understand cross-border care trends
• A patient-centred approach, where patients' perspectives are heard, and they have power over their own treatment data
 |
Co-funded by the European Union.
Project: 101079865 - EuMAR - EU4H-2021-PJ2
"Funded by the European Union. Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union. Neither the European Union nor the granting authority can be held responsible for them."
|